| Name | Description |
|---|---|
| Boiling model | |
| Condensation model | |
| Partial model - Model describing a phase change | |
| Boundary condition for the partial pressure of one of the component of a gas mixture |
 JARA2i.phase.boilingB
JARA2i.phase.boilingB

 .
.  .
.
| Type | Name | Default | Description |
|---|---|---|---|
| Integer | nComp | 1 | Number of components |
| Real | massEnthalpyLRef[nComp] | zeros(nComp) | Specific (per mass) enthalpy of the liquid components at the reference temperature [L2.t-2] |
| Real | molEnthalpyGRef[nComp] | zeros(nComp) | Specific (per mol) enthalpy of the gas components at the reference temperature [M.L2.t-2.mol-1] |
| Real | tempRef | 298 | Reference temperature for the enthalpy [T] |
| Real | CpCoefML[nComp, 7] | Liquid components: coefficients of the specific (per mass) heat capacity Cp unit: L2.t-2.T-1 Cp[i] = CpCoef[i,1] + CpCoef[i,2]*T + ... + CpCoef[i,7]*T**6 | |
| Real | CpCoefNG[nComp, 7] | Vapor components: coefficients of the specific (per mol) heat capacity at constant pressure. Cp unit: M.L2.t-2.T-1.mol-1 Cp[i] = CpCoef[i,1] + CpCoef[i,2]*T + ... + CpCoef[i,7]*T**6 | |
| Real | molecWeigth[nComp] | Molecular weigth of the components [M.mol-1] | |
| Real | eps | 1E-8 | Small constant to avoid by-zero division |
| Real | Kprop | [M.t-1.T-1] |
| Type | Name | Description |
|---|---|---|
| cutLiquidR | inMass | Liquid flow - Connector R. Connector for the liquid flow. |
| cutGasR | outMol | Gas flow - Connector Resistive. |
model boilingB "Boiling model"
extends phaseChgB;
parameter Real Kprop( unit="M.t-1.T-1");
Boolean boiling( start = false, fixed=true) "True: the liquid is boiling
False: the liquid is not boiling";
Boolean noBoiling( start = true, fixed=true) "True : the liquid is not boiling
False: the liquid is boiling";
Real tempBoiling( unit="K") "Temperature of the boiling point";
Real compVaporBoil[ nComp] "Composition (mass fraction of each component)
of the vapor flowing from the liquid, due to the boiling";
Real error( unit="T")
"Difference between the liquid temperature and its boiling temperature";
equation
// Boiling condition
boiling = pre(noBoiling) and not inMass.tempL < tempBoiling or
pre(boiling) and not ( totalMassF < - eps or not ( sum(inMass.massL[i] for i in 1:nComp) > eps));
noBoiling = pre(boiling) and ( totalMassF < - eps or not ( sum(inMass.massL[i] for i in 1:nComp) > eps)) or
pre(noBoiling) and not ( not inMass.tempL < tempBoiling);
// Difference between the liquid temperature and its boiling temperature
error = inMass.tempL - tempBoiling;
// Mass flow due to the boiling
0 = if boiling then totalMassF - Kprop * error else inMass.massLF[1];
if nComp > 1 then
for i in 1:(nComp-1) loop
0 = if boiling then
compVaporBoil[i] - inMass.massLF[i] / ( totalMassF + eps) else
inMass.massLF[i+1];
end for;
end if;
// Direction of the flow
flowIsPosit = true;
end boilingB;
 JARA2i.phase.condensB
JARA2i.phase.condensB

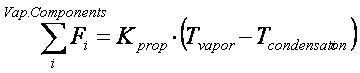 .
.  .
.
| Type | Name | Default | Description |
|---|---|---|---|
| Integer | nComp | 1 | Number of components |
| Real | massEnthalpyLRef[nComp] | zeros(nComp) | Specific (per mass) enthalpy of the liquid components at the reference temperature [L2.t-2] |
| Real | molEnthalpyGRef[nComp] | zeros(nComp) | Specific (per mol) enthalpy of the gas components at the reference temperature [M.L2.t-2.mol-1] |
| Real | tempRef | 298 | Reference temperature for the enthalpy [T] |
| Real | CpCoefML[nComp, 7] | Liquid components: coefficients of the specific (per mass) heat capacity Cp unit: L2.t-2.T-1 Cp[i] = CpCoef[i,1] + CpCoef[i,2]*T + ... + CpCoef[i,7]*T**6 | |
| Real | CpCoefNG[nComp, 7] | Vapor components: coefficients of the specific (per mol) heat capacity at constant pressure. Cp unit: M.L2.t-2.T-1.mol-1 Cp[i] = CpCoef[i,1] + CpCoef[i,2]*T + ... + CpCoef[i,7]*T**6 | |
| Real | molecWeigth[nComp] | Molecular weigth of the components [M.mol-1] | |
| Real | eps | 1E-8 | Small constant to avoid by-zero division |
| Real | Kprop | [mol.t-1.T-1] |
| Type | Name | Description |
|---|---|---|
| cutLiquidR | inMass | Liquid flow - Connector R. Connector for the liquid flow. |
| cutGasR | outMol | Gas flow - Connector Resistive. |
model condensB "Condensation model"
extends phaseChgB;
parameter Real Kprop( unit="mol.t-1.T-1");
protected
Boolean condens( start=false, fixed=true) "True: the liquid is condensing
False: the liquid is not condensing";
Boolean noCondens( start=true, fixed=true) "True : the liquid is not condensing
False: the liquid is condensing";
Real tempCondens( unit="T") "Temperature of the dew point";
Real compLiqCond[ nComp] "Composition (mass fraction of each component)
of the liquid flowing from the vapor, due to the condensation";
Real error( unit="T")
"Difference between the liquid temperature and its dew temperature";
equation
// Condensation
condens = pre(noCondens) and not outMol.tempG > tempCondens or
pre(condens) and not ( totalMolF > eps or not ( sum(outMol.molG[i] for i in 1:nComp) > eps));
noCondens = pre(condens) and ( totalMolF > eps or not ( sum(outMol.molG[i] for i in 1:nComp) > eps)) or
pre(noCondens) and not ( not outMol.tempG > tempCondens);
// Difference between the liquid temperature and its dew temperature
error = outMol.tempG - tempCondens;
// Mass flow due to the condensation
0 = if condens then totalMolF - Kprop * error else outMol.molGF[1];
if nComp > 1 then
for i in 1:(nComp-1) loop
0 = if condens then
compLiqCond[i] - outMol.molGF[i] / ( totalMolF + eps) else
outMol.molGF[i+1];
end for;
end if;
// Flow direction
flowIsPosit = false;
end condensB;
 JARA2i.phase.phaseChgB
JARA2i.phase.phaseChgB

| Type | Name | Default | Description |
|---|---|---|---|
| Integer | nComp | 1 | Number of components |
| Real | massEnthalpyLRef[nComp] | zeros(nComp) | Specific (per mass) enthalpy of the liquid components at the reference temperature [L2.t-2] |
| Real | molEnthalpyGRef[nComp] | zeros(nComp) | Specific (per mol) enthalpy of the gas components at the reference temperature [M.L2.t-2.mol-1] |
| Real | tempRef | 298 | Reference temperature for the enthalpy [T] |
| Real | CpCoefML[nComp, 7] | Liquid components: coefficients of the specific (per mass) heat capacity Cp unit: L2.t-2.T-1 Cp[i] = CpCoef[i,1] + CpCoef[i,2]*T + ... + CpCoef[i,7]*T**6 | |
| Real | CpCoefNG[nComp, 7] | Vapor components: coefficients of the specific (per mol) heat capacity at constant pressure. Cp unit: M.L2.t-2.T-1.mol-1 Cp[i] = CpCoef[i,1] + CpCoef[i,2]*T + ... + CpCoef[i,7]*T**6 | |
| Real | molecWeigth[nComp] | Molecular weigth of the components [M.mol-1] | |
| Real | eps | 1E-8 | Small constant to avoid by-zero division |
| Type | Name | Description |
|---|---|---|
| cutLiquidR | inMass | Liquid flow - Connector R. Connector for the liquid flow. |
| cutGasR | outMol | Gas flow - Connector Resistive. |
partial model phaseChgB
"Partial model - Model describing a phase change"
extends JARA2i.interf.heteroFlow2I;
parameter Real massEnthalpyLRef[nComp]( unit="L2.t-2") = zeros(nComp)
"Specific (per mass) enthalpy of the liquid components at the reference temperature";
parameter Real molEnthalpyGRef[nComp]( unit="M.L2.t-2.mol-1") = zeros(nComp)
"Specific (per mol) enthalpy of the gas components at the reference temperature";
parameter Real tempRef( unit="T") = 298
"Reference temperature for the enthalpy";
parameter Real CpCoefML[nComp,7] "Liquid components: coefficients of the specific (per mass) heat capacity
Cp unit: L2.t-2.T-1
Cp[i] = CpCoef[i,1] + CpCoef[i,2]*T + ... + CpCoef[i,7]*T**6";
parameter Real CpCoefNG[nComp,7] "Vapor components: coefficients of the specific (per mol) heat capacity
at constant pressure. Cp unit: M.L2.t-2.T-1.mol-1
Cp[i] = CpCoef[i,1] + CpCoef[i,2]*T + ... + CpCoef[i,7]*T**6";
parameter Real molecWeigth[nComp]( unit="M.mol-1")
"Molecular weigth of the components";
parameter Real eps = 1E-8 "Small constant to avoid by-zero division";
Real totalMassF( unit="M.t-1")
"Total mass-flow due to the phase change";
Real totalMolF( unit="mol.t-1")
"Total molar-flow due to the phase change";
Real tempF( unit="T")
"Temperature of the flow between the phases";
Real massHeatPhaseChg( unit="L2.t-2")
"Change-of-phase heat per mass unit";
Real massEnthalpyL[ nComp]( unit="L2.t-2")
"Specific (per mass) enthalpy of the liquid components";
Real molEnthalpyL[ nComp]( unit="M.L2.t-2.mol-1")
"Molar enthalpy of the liquid components";
Real molEnthalpyG[ nComp]( unit="M.L2.t-2.mol-1")
"Molar enthalpy of the vapor components";
Real massEnthalpyG[ nComp]( unit="L2.t-2")
"Specific (per mass) enthalpy of the vapor components";
Boolean flowIsPosit "True: flow from liquid to vapor phase
False: flow from vapor to liquid phase";
equation
// Total mass flow due to the phase change. Positive: flow from liquid to vapor phase.
totalMassF = sum(inMass.massLF[i] for i in 1:nComp);
totalMolF = -sum(outMol.molGF[i] for i in 1:nComp);
for i in 1:nComp loop
molecWeigth[i] * outMol.molGF[i] + inMass.massLF[i] = 0;
end for;
// Relationship between the molar enthalpy and the specific (per mass) enthalpy
for i in 1:nComp loop
molecWeigth[i] * molEnthalpyG[i] = massEnthalpyG[i];
molecWeigth[i] * molEnthalpyL[i] = massEnthalpyL[i];
end for;
// Temperature of the change-of-phase flow
tempF = if flowIsPosit then inMass.tempL else outMol.tempG;
// Enthalpy flow associated to the change-of-phase mass flow. Positive: flow from liquid to vapor
inMass.energyLF = if flowIsPosit then
-sum(outMol.molGF[i] * molEnthalpyG[i] for i in 1:nComp) else
sum(inMass.massLF[i] * massEnthalpyL[i] for i in 1:nComp);
outMol.energyGF + inMass.energyLF = 0;
// Specific (per mass) enthalpy of the liquid components
for i in 1:nComp loop
massEnthalpyL[i] = massEnthalpyLRef[i] +
CpCoefML[i,1] * ( tempF - tempRef) +
CpCoefML[i,2] * 1/2 * ( tempF^ 2 - tempRef^ 2) +
CpCoefML[i,3] * 1/3 * ( tempF^ 3 - tempRef^ 3) +
CpCoefML[i,4] * 1/4 * ( tempF^ 4 - tempRef^ 4) +
CpCoefML[i,5] * 1/5 * ( tempF^ 5 - tempRef^ 5) +
CpCoefML[i,6] * 1/6 * ( tempF^ 6 - tempRef^ 6) +
CpCoefML[i,7] * 1/7 * ( tempF^ 7 - tempRef^ 7);
end for;
// Molar enthalpy of the liquid components
for i in 1:nComp loop
molEnthalpyG[i] = molEnthalpyGRef[i] +
CpCoefNG[i,1] * ( tempF - tempRef) +
CpCoefNG[i,2] * 1/2 * ( tempF^ 2 - tempRef^ 2) +
CpCoefNG[i,3] * 1/3 * ( tempF^ 3 - tempRef^ 3) +
CpCoefNG[i,4] * 1/4 * ( tempF^ 4 - tempRef^ 4) +
CpCoefNG[i,5] * 1/5 * ( tempF^ 5 - tempRef^ 5) +
CpCoefNG[i,6] * 1/6 * ( tempF^ 6 - tempRef^ 6) +
CpCoefNG[i,7] * 1/7 * ( tempF^ 7 - tempRef^ 7);
end for;
// Change-of-phase heat per mass unit
// Positive: boiling of a mass unit of liquid mixture
// Negative: condensation of a mass unit of the vapor mixture
massHeatPhaseChg = ( -sum(outMol.molGF[i] * molEnthalpyG[i] for i in 1:nComp) -
sum(inMass.massLF[i] * massEnthalpyL[i] for i in 1:nComp)) / ( totalMassF + eps);
end phaseChgB;
 JARA2i.phase.vapPressB
JARA2i.phase.vapPressB

| Type | Name | Default | Description |
|---|---|---|---|
| Integer | evapComp | Number of the component whose vapor pressure is set | |
| Integer | nComp | 1 | Number of components |
| Real | molEnthalpyRef | 0 | Molar enthalpy of the component at the reference temperature [M.L2.t-2.mol-1] |
| Real | tempRef | 298 | Reference temperature for the enthalpy [T] |
| Real | CpCoefN[1, 7] | Coefficients of the molar heat capacity at constant pressure, of the evapComp component Cp = CpCoefN(1,1) + CpCoefN(1,2)*T + ... | |
| Real | Kprop | 1E4 | Coefficient relating the molar flow of the evapComp component and the pressure difference [mol.L.t.M-1] |
| Real | eps | 1E-4 | Small constant to avoid by-zero division |
| Type | Name | Description |
|---|---|---|
| cutGasR | inMol | Gas flow - Connector Resistive |
| cutReceiver | setPointSignal | Setpoint signals [vaporPressSP, tempEvapSP] |
model vapPressB
"Boundary condition for the partial pressure of one of the component of a gas mixture"
parameter Integer evapComp
"Number of the component whose vapor pressure is set";
extends JARA2i.interf.gasFlow1I;
cutsB.cutReceiver setPointSignal( dim=2, signal={vaporPressSP,tempEvapSP})
"Setpoint signals [vaporPressSP, tempEvapSP]";
Real vaporPressSP( unit="M.L-1.t-2")
"Vapor-pressure setpoint for the evapComp component";
Real tempEvapSP( unit="T")
"Temperature of the component flowing into the control volume";
parameter Real molEnthalpyRef( unit="M.L2.t-2.mol-1") = 0
"Molar enthalpy of the component at the reference temperature";
parameter Real tempRef( unit="T") = 298
"Reference temperature for the enthalpy";
parameter Real CpCoefN[ 1,7] "Coefficients of the molar heat capacity at constant pressure,
of the evapComp component
Cp = CpCoefN(1,1) + CpCoefN(1,2)*T + ...";
parameter Real Kprop( unit="mol.L.t.M-1") = 1E4
"Coefficient relating the molar flow of the evapComp component and the pressure difference";
parameter Real eps = 1E-4 "Small constant to avoid by-zero division";
protected
Real pressErr( unit="M.L-1.t-2")
"Difference between the parcial-pressure setpoint and its actual value";
Real vaporPress( unit="M.L-1.t-2")
"Partial pressure of the evapComp component";
Real aux[nComp+2] "Dummy variables";
Real dummy1 "Dummy variable";
Real dummy2 "Dummy variable";
Real molEnthalpy( unit="M.L2.t-2.mol-1")
"Molar enthalpy of the evapComp component";
Real tempF( unit="T") "Temperature of the evapComp-component flow";
Boolean flowIsPosit "Direction of the evapComp-component flow";
equation
// Vapor pressure of the evapComp component
vaporPress = inMol.pressG * inMol.molG[evapComp] / ( sum(inMol.molG[i] for i in 1:nComp) + eps);
// Difference between the vapor-pressure setpoint and its actual value
pressErr = vaporPressSP + eps - vaporPress;
// Flow of the evapComp component
inMol.molGF[evapComp] = - Kprop * pressErr;
// The flow of all the other components is zero
aux = { dummy1, inMol.molGF, dummy2};
aux[1:evapComp] = 0;
aux[(evapComp+2):(nComp+2)] = 0;
// Molar enthalpy of the evapComp component
for i in 1:nComp loop
molEnthalpy[i] = molEnthalpyRef[i] +
sum( CpCoefN[i,j] * 1/j * ( tempF^ j - tempRef^ j) for j in 1:7);
end for;
// Enthalpy flow
inMol.energyGF = inMol.molGF[evapComp] * molEnthalpy;
// Flow temperture
flowIsPosit = inMol.molGF[evapComp] / ( abs( inMol.molGF[evapComp]) + eps);
tempF = 0.5 * ( 1 + flowIsPosit) * inMol.tempG - 0.5 * ( flowIsPosit -1) * tempEvapSP;
end vapPressB;